Aminophosphine Chemistry
Aminophosphine Chemistry
Aminophosphines
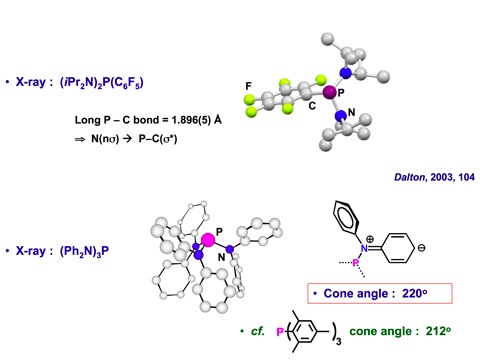
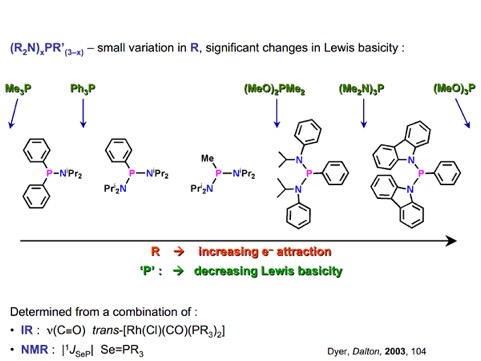
Alkoxy- and aryloxy-functionalised phosphines, have long been used as alternatives to C-substituted phosphines, which are common ligands in transition metal chemistry. Within the group we are developing the coordination chemistry and applications in catalysis of amino-phosphines, RxP(NR’2)3-x (x = 0-2), compounds that have received contrastingly little attention.1,2 They are easy to prepare through reaction of a secondary amine with a halophosphine. This allows extensive structural and electronic diversity to be achieved, in a systematic way, through judicious choice of NR’2 unit. In particular, significant steric bulk can be located about the phosphorus centre (e.g.1, cone angle 220º); bulky ligands find application in a number of areas of catalysis. Furthermore, the chemistry of aminophosphines with their polar P-N bonds is extremely rich, both in and out of the coordination sphere of metals, opening up a range of unique, but synthetically useful transformations.
References
1. P. W. Dyer, J. Fawcett, M. J. Hanton, R. D. W. Kemmitt, R. Padda, D. R. Russell, and N. Singh, J. Chem. Soc. Dalton Trans., 2003, 104-113.
2. E. Despagnet, K. Miqueu, H. Gornitzka, P. W. Dyer, D. Bourissou, and G. Bertrand, J. Am. Chem. Soc., 2002, 124, 11834-11835.
3. F. Dahan, P. W. Dyer, M. J. Hanton, M. Jones, D. M. P. Mingos, A. J. P. White, D. J. Williams, and A.-M. Williamson, Eur. J. Inorg. Chem., 2002, 737-742.
4. A. Baceiredo, G. Bertrand, F. Dahan, P. W. Dyer,J. Fawcett, N. Griep-Ramin, O. Guerret, M. J. Hanton, D. R. Russell and A-M. Williamson, New J. Chem., 2001, 25, 591-596.
